Literature Melting Point Of Naphthalene
| |||
 | |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Naphthalene[2] | |||
| Systematic IUPAC name Bicyclo[4.4.0]deca-1,3,5,7,9-pentaene | |||
| Other names white tar, camphor tar, tar camphor, naphthalin, naphthaline, antimite, albocarbon, hexalene, mothballs, moth flakes[1] | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| Beilstein Reference | 1421310 | ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.001.863 | ||
| EC Number |
| ||
| Gmelin Reference | 3347 | ||
| KEGG |
| ||
| PubChem CID |
| ||
| RTECS number |
| ||
| UNII |
| ||
| CompTox Dashboard (EPA) |
| ||
| InChI
| |||
| SMILES
| |||
| Properties | |||
| Chemical formula | C 10 H 8 | ||
| Molar mass | 128.174 m·mol−1 | ||
| Appearance | White solid crystals/ flakes | ||
| Odor | Stiff odour of coal tar | ||
| Density | i.145 chiliad/cmthree (15.v °C)[3] 1.0253 g/cm3 (20 °C)[4] 0.9625 g/cm3 (100 °C)[3] | ||
| Melting betoken | 78.2 °C (172.8 °F; 351.3 M) 80.26 °C (176.47 °F; 353.41 Grand) at 760 mmHg[4] | ||
| Boiling bespeak | 217.97 °C (424.35 °F; 491.12 G) at 760 mmHg[three] [4] | ||
| Solubility in h2o | 19 mg/L (ten °C) 31.6 mg/L (25 °C) 43.9 mg/Fifty (34.five °C) 80.9 mg/L (l °C)[4] 238.i mg/L (73.4 °C)[5] | ||
| Solubility | Soluble in alcohols, liquid ammonia, Carboxylic acids, C6Hhalf dozen, SOtwo,[5] CCl4, CStwo, toluene, aniline[six] | ||
| Solubility in ethanol | 5 grand/100 g (0 °C) 11.iii g/100 g (25 °C) 19.5 g/100 g (40 °C) 179 g/100 g (lxx °C)[6] | ||
| Solubility in acetic acrid | 6.eight g/100 k (6.75 °C) 13.i g/100 g (21.5 °C) 31.one g/100 g (42.v °C) 111 m/100 g (lx °C)[half-dozen] | ||
| Solubility in chloroform | 19.5 grand/100 g (0 °C) 35.5 1000/100 1000 (25 °C) 49.5 g/100 g (40 °C) 87.2 1000/100 grand (70 °C)[6] | ||
| Solubility in hexane | 5.5 g/100 g (0 °C) 17.v 1000/100 g (25 °C) 30.8 g/100 1000 (40 °C) 78.8 g/100 g (70 °C)[half-dozen] | ||
| Solubility in butyric acid | xiii.six g/100 m (6.75 °C) 22.1 1000/100 1000 (21.5 °C) 131.6 g/100 grand (sixty °C)[half-dozen] | ||
| log P | iii.34[4] | ||
| Vapor pressure | 8.64 Pa (20 °C) 23.half dozen Pa (30 °C) 0.93 kPa (80 °C)[v] 2.5 kPa (100 °C)[7] | ||
| Henry'southward law | 0.42438 Fifty·atm/mol[4] | ||
| Magnetic susceptibility (χ) | -91.9·10−half-dozen cm3/mol | ||
| Thermal conductivity | 98 kPa: 0.1219 Due west/thousand·K (372.22 K) 0.1174 W/m·Thou (400.22 K) 0.1152 W/thou·Grand (418.37 1000) 0.1052 W/g·One thousand (479.72 M)[8] | ||
| Refractive alphabetize (n D) | 1.5898[4] | ||
| Viscosity | 0.964 cP (80 °C) 0.761 cP (100 °C) 0.217 cP (150 °C)[nine] | ||
| Structure | |||
| Crystal construction | Monoclinic[ten] | ||
| Space group | P21/b[x] | ||
| Point grouping | C 5 2h [x] | ||
| Lattice abiding | a = eight.235 Å, b = 6.003 Å, c = eight.658 Å[ten] α = 90°, β = 122.92°, γ = 90° | ||
| Thermochemistry | |||
| Rut capacity (C) | 165.72 J/mol·One thousand[4] | ||
| Std molar | 167.39 J/mol·K[four] [seven] | ||
| Std enthalpy of | 78.53 kJ/mol[iv] | ||
| Gibbs free free energy (Δf G ⦵) | 201.585 kJ/mol[four] | ||
| Std enthalpy of | -5156.three kJ/mol[4] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Primary hazards | Flammable, sensitizer, possible carcinogen.[12] Dust can form explosive mixtures with air | ||
| GHS labelling: | |||
| Pictograms |     [xi] [xi] | ||
| Indicate discussion | Danger | ||
| Take a chance statements | H228, H302, H351, H410 [eleven] | ||
| Precautionary statements | P210, P273, P281, P501 [eleven] | ||
| NFPA 704 (fire diamond) | ii 2 0 | ||
| Flash point | 80 °C (176 °F; 353 K)[11] | ||
| Autoignition | 525 °C (977 °F; 798 K)[xi] | ||
| Explosive limits | 5.nine%[11] | ||
| Threshold limit value (TLV) | 10 ppm[iv] (TWA), xv ppm[iv] (STEL) | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 1800 mg/kg (rat, oral) 490 mg/kg (rat, intravenous) 1200 mg/kg (guinea grunter, oral) 533 mg/kg (mouse, oral)[14] | ||
| NIOSH (U.s. health exposure limits): | |||
| PEL (Permissible) | TWA 10 ppm (l mg/m3)[xiii] | ||
| REL (Recommended) | TWA 10 ppm (fifty mg/m3) ST 15 ppm (75 mg/g3)[thirteen] | ||
| IDLH (Immediate danger) | 250 ppm[13] | ||
| Except where otherwise noted, data are given for materials in their standard land (at 25 °C [77 °F], 100 kPa). Infobox references | |||
Naphthalene is an organic chemical compound with formula C
ten H
8 . Information technology is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic aroma that is detectable at concentrations as low every bit 0.08 ppm by mass.[15] As an effluvious hydrocarbon, naphthalene'southward structure consists of a fused pair of benzene rings. It is best known equally the master ingredient of traditional mothballs.
History [edit]
In the early on 1820s, two split reports described a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd cited these ii disclosures and then described many of this substance's properties and the means of its production. He proposed the name naphthaline, as information technology had been derived from a kind of naphtha (a broad term encompassing any volatile, combustible liquid hydrocarbon mixture, including coal tar).[16] Naphthalene'southward chemical formula was determined past Michael Faraday in 1826. The structure of two fused benzene rings was proposed by Emil Erlenmeyer in 1866,[17] and confirmed by Carl Gräbe three years afterwards.[18]
Physical backdrop [edit]

Bicyclo[half dozen.2.0]decapentaene
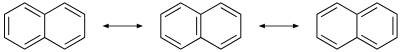
A naphthalene molecule can be viewed equally the fusion of a pair of benzene rings. (In organic chemistry, rings are fused if they share two or more atoms.) As such, naphthalene is classified every bit a benzenoid polycyclic effluvious hydrocarbon (PAH).
The eight carbon atoms that are not shared past the two rings conduct one hydrogen atom each. For purpose of the standard IUPAC classification of derived compounds, those 8 atoms are numbered 1 through 8 in sequence effectually the perimeter of the molecule, starting with a carbon cantlet adjacent to a shared ane. The shared carbon atoms are labeled 4a (betwixt 4 and 5) and 8a (between 8 and one).
Molecular geometry [edit]
The molecule is planar, like benzene. Unlike benzene, the carbon–carbon bonds in naphthalene are not of the aforementioned length. The bonds C1−C2, C3−C4, C5−C6 and C7−C8 are near 1.37 Å (137 pm) in length, whereas the other carbon–carbon bonds are about 1.42 Å (142 pm) long. This deviation, established past X-ray diffraction,[nineteen] is consequent with the valence bond model in naphthalene and in particular, with the theorem of cross-conjugation. This theorem would describe naphthalene as an aromatic benzene unit bonded to a diene but non extensively conjugated to it (at to the lowest degree in the ground state), which is consistent with two of its three resonance structures.
Because of this resonance, the molecule has bilateral symmetry across the airplane of the shared carbon pair, likewise as across the plane that bisects bonds C2-C3 and C6-C7, and beyond the plane of the carbon atoms. Thus there are two sets of equivalent hydrogen atoms: the alpha positions, numbered 1, 4, 5, and 8, and the beta positions, 2, three, half-dozen, and 7. Two isomers are then possible for mono-substituted naphthalenes, corresponding to commutation at an alpha or beta position. Bicyclo[6.2.0]decapentaene is a structural isomer with a fused 4–8 ring system[20] and azulene is another, with a fused 5-7 ring system.
The point group symmetry of naphthalene is D2h .
Electrical electrical conductivity [edit]
Pure crystalline naphthalene is a moderate insulator at room temperature, with resistivity of about ten12 Ω m. The resistivity drops more than a thousandfold on melting, to about 4 × tenviii Ω m. Both in the liquid and in the solid, the resistivity depends on temperature as ρ = ρ 0 exp(Due east/(k T)), where ρ 0 (Ω m) and E (eV) are constant parameters, 1000 is Boltzmann's constant (8.617×ten−v eV/Grand), and T is absolute temperature (Thousand). The parameter E is 0.73 in the solid. Even so, the solid shows semiconducting character below 100 K.[21] [22]
Chemical properties [edit]
Reactions with electrophiles [edit]
In electrophilic aromatic substitution reactions, naphthalene reacts more readily than benzene. For example, chlorination and bromination of naphthalene proceeds without a catalyst to give 1-chloronaphthalene and i-bromonaphthalene, respectively. Likewise, whereas both benzene and naphthalene can exist alkylated using Friedel–Crafts reactions, naphthalene tin can also be hands alkylated by reaction with alkenes or alcohols, using sulfuric or phosphoric acid catalysts.
In terms of regiochemistry, electrophiles set on at the alpha position. The selectivity for alpha over beta substitution can exist rationalized in terms of the resonance structures of the intermediate: for the blastoff exchange intermediate, seven resonance structures tin can be fatigued, of which four preserve an aromatic band. For beta substitution, the intermediate has only six resonance structures, and only 2 of these are aromatic. Sulfonation gives the "blastoff" product naphthalene-1-sulfonic acid as the kinetic product but naphthalene-2-sulfonic acid as the thermodynamic product. The 1-isomer forms predominantly at 25 °C, and the 2-isomer at 160 °C. Sulfonation to requite the one- and 2-sulfonic acrid occurs readily:
- H
2 And then
four + C
ten H
8 → C
10 H
7 −And so
3 H + H
2 O
Farther sulfonation give di-, tri-, and tetrasulfonic acids.
Lithiation [edit]
Coordinating to the synthesis of phenyllithium is the conversion of ane-bromonathalene to 1-lithionaphthalene, by lithium–halogen substitution:
- CtenH7Br + BuLi → CxH7Li + BuBr
The resulting lithionaphthalene undergoes a second lithiation, in contrast to the behavior of phenyllithium. These 1,eight-dilithio derivatives are precursors to a host of peri-naphthalene derivatives.[23]
Reduction and oxidation [edit]
With alkali metals, naphthalene forms the dark blue-green radical anion salts such as sodium naphthalene, Na+CtenH −
viii . The naphthalene anions are strong reducing agents.
Naphthalene tin be hydrogenated under high pressure level in the presence of metal catalysts to give 1,2,iii,iv-tetrahydronaphthalene(C
x H
12 ), also known every bit tetralin. Further hydrogenation yields decahydronaphthalene or decalin (C
10 H
18 ).
Oxidation with O
2 in the presence of vanadium pentoxide as catalyst gives phthalic anhydride:
- CxH8 + iv.5 Oii → C6H4(CO)iiO + 2 CO2 + 2 HiiO
This reaction is the basis of the chief use of naphthalene. Oxidation can also be effected using conventional stoichiometric chromate or permanganate reagents.
Product [edit]

Nigh naphthalene is derived from coal tar. From the 1960s until the 1990s, pregnant amounts of naphthalene were also produced from heavy petroleum fractions during petroleum refining, but today petroleum-derived naphthalene represents only a minor component of naphthalene production.
Naphthalene is the most abundant single component of coal tar. Although the composition of coal tar varies with the coal from which it is produced, typical coal tar is about 10% naphthalene by weight. In industrial practice, distillation of coal tar yields an oil containing nearly l% naphthalene, along with twelve other aromatic compounds. This oil, after being done with aqueous sodium hydroxide to remove acidic components (chiefly various phenols), and with sulfuric acid to remove bones components, undergoes partial distillation to isolate naphthalene. The crude naphthalene resulting from this process is about 95% naphthalene past weight. The chief impurities are the sulfur-containing effluvious compound benzothiophene (< two%), indane (0.ii%), indene (< 2%), and methylnaphthalene (< 2%). Petroleum-derived naphthalene is ordinarily purer than that derived from coal tar. Where required, crude naphthalene can be further purified past recrystallization from whatever of a diverseness of solvents, resulting in 99% naphthalene by weight, referred to equally 80 °C (melting bespeak). Approximately 1.3M tons are produced annually.[24]
In Due north America, the coal tar producers are Koppers Inc., Ruetgers Canada Inc. and Recochem Inc., and the primary petroleum producer is Monument Chemic Inc. In Western Europe the well-known producers are Koppers, Ruetgers, and Deza. In Eastern Europe, naphthalene is produced by a multifariousness of integrated metallurgy complexes (Severstal, Evraz, Mechel, MMK) in Russia, dedicated naphthalene and phenol makers INKOR, Yenakievsky Metallurgy establish in Ukraine and ArcelorMittal Temirtau in Kazakhstan.
Other sources and occurrences [edit]
Aside from coal tar, trace amounts of naphthalene are produced by magnolias and some species of deer, equally well as the Formosan subterranean termite, possibly produced by the termite as a repellant against "ants, poisonous fungi and nematode worms."[25] Some strains of the endophytic mucus Muscodor albus produce naphthalene amid a range of volatile organic compounds, while Muscodor vitigenus produces naphthalene almost exclusively.[26]
Naphthalene in the interstellar medium [edit]
Naphthalene has been tentatively detected in the interstellar medium in the management of the star Cernis 52 in the constellation Perseus.[27] [28] More than xx% of the carbon in the universe may be associated with polyaromatic hydrocarbons, including naphthalene.[29]
Protonated cations of naphthalene (C
x H +
9 ) are the source of office of the spectrum of the Unidentified Infrared Emissions (UIRs). Protonated naphthalene differs from neutral naphthalene (e.k. that used in mothballs) in that information technology has an additional hydrogen atom. The UIRs from "naphthalene cation" (C
ten H +
9 ) have been observed by astronomers. This research has been publicized as "mothballs in infinite."[thirty]
Uses [edit]
Naphthalene is used mainly as a forerunner to other chemicals. The single largest utilize of naphthalene is the industrial production of phthalic anhydride, although more than phthalic anhydride is made from o-xylene. Many azo dyes are produced from naphthalene, and and so is the insecticide 1-naphthyl-N-methylcarbamate (carbaryl). Other useful agrichemicals include naphthoxyacetic acids.
Hydrogenation of naphthalene gives tetralin, which is used as a hydrogen-donor solvent.[24]
Alkylation of naphthalene with propylene gives a mixture of diisopropylnaphthalenes, which are useful as nonvolatile liquids used in inks.[24]
Naphthalenesulfonic acids and sulfonates [edit]
Many naphthalenesulfonic acids and sulfonates are useful. Alkyl naphthalene sulfonate are surfactants, The aminonaphthalenesulfonic acids, naphthalenes substituted with ethers and sulfonic acids, are intermediates in the preparation of many constructed dyes. The hydrogenated naphthalenes tetrahydronaphthalene (tetralin) and decahydronaphthalene (decalin) are used as low-volatility solvents. Naphthalene sulfonic acids are likewise used in the synthesis of one-naphthol and ii-naphthol, precursors for various dyestuffs, pigments, condom processing chemicals and other chemicals and pharmaceuticals.[24]
Naphthalene sulfonic acids are used in the manufacture of naphthalene sulfonate polymer plasticizers (dispersants), which are used to produce concrete and plasterboard (wallboard or drywall). They are as well used equally dispersants in synthetic and natural rubbers, and as tanning agents (syntans) in leather industries, agricultural formulations (dispersants for pesticides), dyes and as a dispersant in lead–acid battery plates.
Naphthalene sulfonate polymers are produced by treating naphthalenesulfonic acid with formaldehyde, followed by neutralization with sodium hydroxide or calcium hydroxide. These products are commercially sold equally superplasticizers for the production of high strength concrete.
Laboratory uses [edit]
Molten naphthalene provides an excellent solubilizing medium for poorly soluble effluvious compounds. In many cases it is more efficient than other loftier-boiling solvents, such as dichlorobenzene, benzonitrile, nitrobenzene and durene. The reaction of C60 with anthracene is conveniently conducted in refluxing naphthalene to requite the 1:one Diels–Alder adduct.[31] The aromatization of hydroporphyrins has been accomplished using a solution of DDQ in naphthalene.[32]
Wetting agent and surfactant [edit]
Alkyl naphthalene sulfonates (ANS) are used in many industrial applications as nondetergent wetting agents that effectively disperse colloidal systems in aqueous media. The major commercial applications are in the agricultural chemical manufacture, which uses ANS for wettable powder and wettable granular (dry out-flowable) formulations, and the textile and fabric industry, which utilizes the wetting and defoaming backdrop of ANS for bleaching and dyeing operations.
Equally a fumigant [edit]
Naphthalene has been used as a household fumigant. It was once the main ingredient in mothballs, although its use has largely been replaced in favor of alternatives such as 1,four-dichlorobenzene. In a sealed container containing naphthalene pellets, naphthalene vapors build upwardly to levels toxic to both the adult and larval forms of many moths that attack textiles. Other fumigant uses of naphthalene include employ in soil every bit a fumigant pesticide, in cranium spaces to repel animals and insects, and in museum storage-drawers and cupboards to protect the contents from attack past insect pests.
Naphthalene is a repellent to opossums.[33] [34]
Other uses [edit]
It is used in pyrotechnic special effects such as the generation of black smoke and faux explosions.[35] Information technology is used to create bogus pores in the manufacture of high-porosity grinding wheels. In the past, naphthalene was administered orally to impale parasitic worms in livestock. Naphthalene and its alkyl homologs are the major constituents of creosote. Naphthalene is used in engineering to study heat transfer using mass sublimation.
It has been proposed as an alternative propellant for cold gas satellite thrusters.[36] [37]
Health effects [edit]
Exposure to large amounts of naphthalene may damage or destroy ruddy blood cells, about normally in people with the inherited condition known as glucose-half-dozen-phosphate dehydrogenase (G6PD) deficiency,[38] which over 400 million people suffer from. Humans, in particular children, have developed the condition known as hemolytic anemia, after ingesting mothballs or deodorant blocks containing naphthalene. Symptoms include fatigue, lack of appetite, restlessness, and pale peel. Exposure to big amounts of naphthalene may cause defoliation, nausea, vomiting, diarrhea, blood in the urine, and jaundice (yellow coloration of the pare due to dysfunction of the liver).[39]
The United states of america National Toxicology Plan (NTP) held an experiment where male and female rats and mice were exposed to naphthalene vapors on weekdays for ii years.[40] Both male and female rats exhibited testify of carcinogenesis with increased incidences of adenoma and neuroblastoma of the nose. Female mice exhibited some testify of carcinogenesis based on increased incidences of alveolar and bronchiolar adenomas of the lung, while male mice exhibited no evidence of carcinogenesis.
The International Agency for Enquiry on Cancer (IARC)[41] classifies naphthalene as mayhap carcinogenic to humans and animals (Group 2B). The IARC also points out that astute exposure causes cataracts in humans, rats, rabbits, and mice; and that hemolytic anemia (described in a higher place) can occur in children and infants after oral or inhalation exposure or after maternal exposure during pregnancy. Nether California'southward Proposition 65, naphthalene is listed as "known to the State to cause cancer".[42] A probable machinery for the carcinogenic effects of mothballs and some types of air fresheners containing naphthalene has been identified.[43] [44]
Regulation [edit]
United states of america authorities agencies have set occupational exposure limits to naphthalene exposure. The Occupational Safety and Health Administration has set a permissible exposure limit at x ppm (l mg/mthree) over an viii-hour fourth dimension-weighted boilerplate. The National Institute for Occupational Prophylactic and Wellness has ready a recommended exposure limit at 10 ppm (50 mg/m3) over an eight-hr time-weighted average, every bit well as a brusque-term exposure limit at 15 ppm (75 mg/m3).[45] Naphthalene's minimum odor threshold is 0.084 ppm for humans.[46]
Mothballs and other products containing naphthalene accept been banned within the EU since 2008.[47] [48]
In Mainland china, the use of naphthalene in mothballs is forbidden.[49] Danger to human health and the common use of natural camphor are cited as reasons for the ban.
Naphthalene derivatives [edit]
The partial list of naphthalene derivatives includes the following compounds:
| Name | Chemic formula | Molar mass [yard/mol] | Melting point [°C] | Humid bespeak [°C] | Density [chiliad/cmthree] | Refractive index |
|---|---|---|---|---|---|---|
| 1-Naphthoic acid | CxiH8O2 | 172.18 | 157 | 300 | – | |
| ane-Naphthoyl chloride | C11H7ClO | 190.63 | 16–19 | 190 (35 Torr) | 1.265 | one.6552 |
| 1-Naphthol | C10H8O | 144,17 | 94–96 | 278 | 1.224 | – |
| 1-Naphthaldehyde | C11H8O | 156,18 | 1–2 | 160 (xv Torr) | ||
| ane-Nitronaphthalene | CtenHviiNO2 | 173.17 | 53–57 | 340 | 1.22 | – |
| 1-Fluoronaphthalene | CtenH7F | 146.16 | −19 | 215 | one.323 | 1.593 |
| one-Chloronaphthalene | C10HviiCl | 162.62 | −6 | 259 | 1.194 | 1.632 |
| 2-Chloronaphthalene | C10H7Cl | 162.62 | 59.5 | 256 | ane.138 | 1.643 |
| 1-Bromonaphthalene | C10H7Br | 207.07 | −two | 279 | 1.489 | one.670 |
| 1,two,seven-Trimethylnaphthalene (Sapotalin) | C13H14 | 170.25 | 143 | 128 | 0.987 |
See also [edit]
- Camphor
- Dialin, Tetralin, Decalin
- List of interstellar and circumstellar molecules
- Mothballs
- ane-Naphthol, two-Naphthol
- Sodium naphthalenide
- Wagner-Jauregg reaction (classic naphthalene synthesis)
References [edit]
- ^ Naphthalene: trade names
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blueish Volume). Cambridge: The Royal Society of Chemistry. 2014. pp. 13, 35, 204, 207, 221–222, 302, 457, 461, 469, 601, 650. doi:10.1039/9781849733069-FP001. ISBN978-0-85404-182-four.
- ^ a b c "Ambient H2o Quality Criteria for Naphthalene" (PDF). United States Ecology Protection Bureau. 2014-04-23. Retrieved 2014-06-21 .
- ^ a b c d eastward f one thousand h i j g fifty m due north Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN978-i-4200-9084-0.
- ^ a b c Anatolievich, Kiper Ruslan. "naphthalene". chemister.ru . Retrieved 2014-06-21 .
- ^ a b c d e f Seidell, Atherton; Linke, William F. (1919). Solubility of Inorganic and Organic Compounds (2nd ed.). New York: D. Van Nostrand Company. pp. 443–446.
- ^ a b Naphthalene in Linstrom, Peter J.; Mallard, William Thou. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (Md) (retrieved 2014-05-24)
- ^ "Thermal Conductivity of Naphthalene". DDBST GmbH. DDBST GmbH. Retrieved 2014-06-21 .
- ^ "Dynamic Viscosity of Naphthalene". DDBST GmbH. DDBST GmbH. Retrieved 2014-06-21 .
- ^ a b c d Douglas, Bodie Eastward.; Ho, Shih-Ming (2007). Structure and Chemistry of Crystalline Solids. New York: Springer Science+Business Media, Inc. p. 288. ISBN978-0-387-26147-8.
- ^ a b c d due east f Sigma-Aldrich Co., Naphthalene.
- ^ Naphthalene carcinogenicity
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0439". National Constitute for Occupational Safe and Health (NIOSH).
- ^ "Naphthalene". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safe and Health (NIOSH).
- ^ Amoore JE, Hautala E (1983). "Odour equally an assistance to chemical prophylactic: Odor thresholds compared with threshold limit values and volatiles for 214 industrial chemicals in air and h2o dilution". J Appl Toxicology. 3 (6): 272–290. doi:10.1002/jat.2550030603. PMID 6376602. S2CID 36525625.
- ^ John Kidd (1821). "Observations on Naphthalene, a peculiar substance resembling a concrete essential oil, which is produced during the decomposition of coal tar, past exposure to a red heat". Philosophical Transactions. 111: 209–221. doi:ten.1098/rstl.1821.0017. S2CID 97798085.
- ^ Emil Erlenmeyer (1866). "Studien über die south. g. aromatischen Säuren". Annalen der Chemie und Pharmacie. 137 (3): 327–359. doi:ten.1002/jlac.18661370309.
- ^ C. Graebe (1869) "Ueber die Constitution des Naphthalins" (On the structure of naphthalene), Annalen der Chemie und Pharmacie, 149 : 20–28.
- ^ Cruickshank, D. W. J.; Sparks, R. A. (18 October 1960). "Experimental and Theoretical Determinations of Bond Lengths in Naphthalene, Anthracene and Other Hydrocarbons". Proceedings of the Purple Society A: Mathematical, Physical and Applied science Sciences. 258 (1293): 270–285. Bibcode:1960RSPSA.258..270C. doi:x.1098/rspa.1960.0187. S2CID 96765335.
- ^ Dieter Cremer; Thomas Schmidt; Charles W. Bock (1985). "Theoretical determination of molecular structure and conformation. 14. Is bicyclo[6.ii.0]decapentaene aromatic or antiaromatic?". J. Org. Chem. 50 (15): 2684–2688. doi:ten.1021/jo00215a018.
- ^ Bornmann John A (1962). "Semiconductivity of Naphthalene". Periodical of Chemical Physics. 36 (6): 1691–1692. Bibcode:1962JChPh..36.1691B. doi:10.1063/ane.1732805.
- ^ Schein L. B., Duke C. B., McGhie A. R. (1978). "Observation of the Ring-Hopping Transition for Electrons in Naphthalene". Physical Review Letters. twoscore (iii): 197–200. Bibcode:1978PhRvL..40..197S. doi:10.1103/PhysRevLett.40.197.
{{cite periodical}}: CS1 maint: multiple names: authors list (link) - ^ van Soolingen J, de Lang RJ, den Besten R, et al. (1995). "A uncomplicated process for the training of 1,8-bis(diphenylphosphino)naphthalene". Constructed Communications. 25 (11): 1741–1744. doi:ten.1080/00397919508015858.
- ^ a b c d Gerd Collin, Hartmut Höke, Helmut Greim (2003). "Naphthalene and Hydronaphthalenes". Ullmann'southward Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
{{cite encyclopedia}}: CS1 maint: uses authors parameter (link). - ^ "Termite 'mothball' keep insects at bay". Sci/Tech. BBC News. Apr 8, 1998.
- ^ Daisy BH, Strobel GA, Castillo U, et al. (November 2002). "Naphthalene, an insect repellent, is produced by Muscodor vitigenus, a novel endophytic fungus". Microbiology. 148 (Pt eleven): 3737–41. doi:10.1099/00221287-148-xi-3737. PMID 12427963.
- ^ "Interstellar Space Molecules That Help Form Basic Life Structures Identified". Scientific discipline Daily. September 2008.
- ^ Iglesias-Groth, S.; et al. (2008-09-20), "Evidence for the Naphthalene Cation in a Region of the Interstellar Medium with Dissonant Microwave Emission", The Astrophysical Periodical Letters, 685 (ane): L55–L58, arXiv:0809.0778, Bibcode:2008ApJ...685L..55I, doi:10.1086/592349, S2CID 17190892 - This spectral assignment has not been independently confirmed, and is described past the authors as "tentative" (page L58).
- ^ Hoover, Rachel (February 21, 2014). "Need to Track Organic Nano-Particles Across the Universe? NASA's Got an App for That". NASA . Retrieved February 22, 2014.
- ^ "Mothballs in Space". Astrobiology Magazine . Retrieved Dec 25, 2008.
- ^ K. Komatsua; Y. Murataa; Northward. Sugitaa; et al. (1993). "Employ of naphthalene as a solvent for selective formation of the 1:1 Diels–Alder adduct of Csixty with anthracene". Tetrahedron Letters. 34 (52): 8473–8476. doi:10.1016/S0040-4039(00)61362-X.
- ^ G.A. Filatov; A.V. Cheprakov (2011). "The synthesis of new tetrabenzo- and tetranaphthoporphyrins via the addition reactions of 4,7-dihydroisoindole". Tetrahedron. 67 (19): 3559–3566. doi:x.1016/j.tet.2011.01.052.
- ^ "Summary of Possum Repellent Study". Archived from the original on September 28, 2013.
- ^ "Removing a possum from your roof | NSW Environment & Heritage". Archived from the original on 2013-09-27. Retrieved 2013-09-07 .
- ^ Lu, Pei; Li, Caiting; Zeng, Guangming; et al. (2012-01-15). "Research on soot of black smoke from ceramic furnace flue gas: Characterization of soot". Journal of Hazardous Materials. 199–200: 272–281. doi:10.1016/j.jhazmat.2011.11.004. ISSN 0304-3894. PMID 22138172.
- ^ Tsifakis, Dimitrios; Charles, Christine; Boswell, Rod (2020-09-23). "Naphthalene as a Cubesat Cold Gas Thruster Propellant". Frontiers in Physics. 8: 389. Bibcode:2020FrP.....8..389T. doi:10.3389/fphy.2020.00389. hdl:1885/229663.
- ^ "New propulsion system using the fundamental ingredient in moth balls could propel satellites through space". Australian Broadcasting Corporation. eight December 2021. Retrieved Dec 11, 2021.
- ^ Santucci K, Shah B (January 2000). "Association of naphthalene with acute hemolytic anemia". Acad Emerg Med. 7 (1): 42–7. doi:10.1111/j.1553-2712.2000.tb01889.ten. PMID 10894241.
- ^ MedlinePlus Encyclopedia: Naphthalene poisoning
- ^ "NTP Technical Reports 410 and 500". NTP Technical Reports 410 and 500, bachelor from NTP: Long-Term Abstracts & Reports. Archived from the original on October 24, 2004. Retrieved March half-dozen, 2005.
- ^ IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Monographs on the Evaluation of Carcinogenic Risks to Humans, Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene, Vol. 82 (2002) (p. 367). ISBN9789283212829 . Retrieved December 25, 2008.
- ^ Suggestion 65 Archived 2019-07-29 at the Wayback Machine, Part of Ecology Wellness Hazard Cess
- ^ "Scientists May Have Solved Mystery Of Carcinogenic Mothballs", Physorg.com, June 20, 2006.
- ^ "Mothballs, air fresheners and cancer". Environmental Health Association of Nova Scotia. Ecology Health Association of Nova Scotia. Retrieved 24 May 2013.
- ^ "CDC - NIOSH Pocket Guide to Chemic Hazards - Naphthalene". Cdc.gov . Retrieved 6 March 2022.
- ^ "Naphthalene" (PDF). Epa.gov . Retrieved 6 March 2022.
- ^ Alderson, Andrew (15 Nov 2008). "Holy straight bananas – now the Eurocrats are banning moth assurance". The Telegraph. Archived from the original on 2022-01-12. Retrieved 2013-11-23 .
- ^ Gray, Kerrina (17 Nov 2013). "Quango warned confronting apply of poisonous moth assurance". Your Local Guardian. Newsquest (London) Ltd. Retrieved 2012-11-23 .
- ^ 国务院经贸办、卫生部关于停止生产和销售萘丸提倡使用樟脑制品的通知(国经贸调(1993)64号)
External links [edit]
- Naphthalene—National Pesticide Information Eye
- Naphthalene—EPA Air Toxics Web Site
- Naphthalene (PIM 363)—mostly on toxicity of naphthalene
- Naphthalene—CDC – NIOSH Pocket Guide to Chemic Hazards
- Naphthalene in the Pesticide Properties DataBase (PPDB)
Literature Melting Point Of Naphthalene,
Source: https://en.wikipedia.org/wiki/Naphthalene
Posted by: hamelwithris.blogspot.com





0 Response to "Literature Melting Point Of Naphthalene"
Post a Comment