Are Hexane And Benzene Miscible
Affiliate ix.2: Solubility and Structure
- Page ID
- 42100
Learning Objective
- To sympathise the human relationship between solubility and molecular structure.
When a solute dissolves, its individual atoms, molecules, or ions interact with the solvent, become solvated, and are able to diffuse independently throughout the solution (part (a) in Figure nine.ii.1). This is not, even so, a unidirectional procedure. If the molecule or ion happens to collide with the surface of a particle of the undissolved solute, information technology may attach to the particle in a process called crystallization. Dissolution and crystallization continue as long as backlog solid is present, resulting in a dynamic equilibrium analogous to the equilibrium that maintains the vapor pressure level of a liquid. (For more information virtually vapor pressure, meet Section vii.iv) We can stand for these opposing processes as follows:
\( solute+solvent \rightleftharpoons crystallization \; dissolution \; solution \tag{9.two.ane} \)
Although the terms precipitation and crystallization are both used to describe the separation of solid solute from a solution, crystallization refers to the formation of a solid with a well-defined crystalline structure, whereas atmospheric precipitation refers to the formation of any solid phase, often one with very small-scale particles.
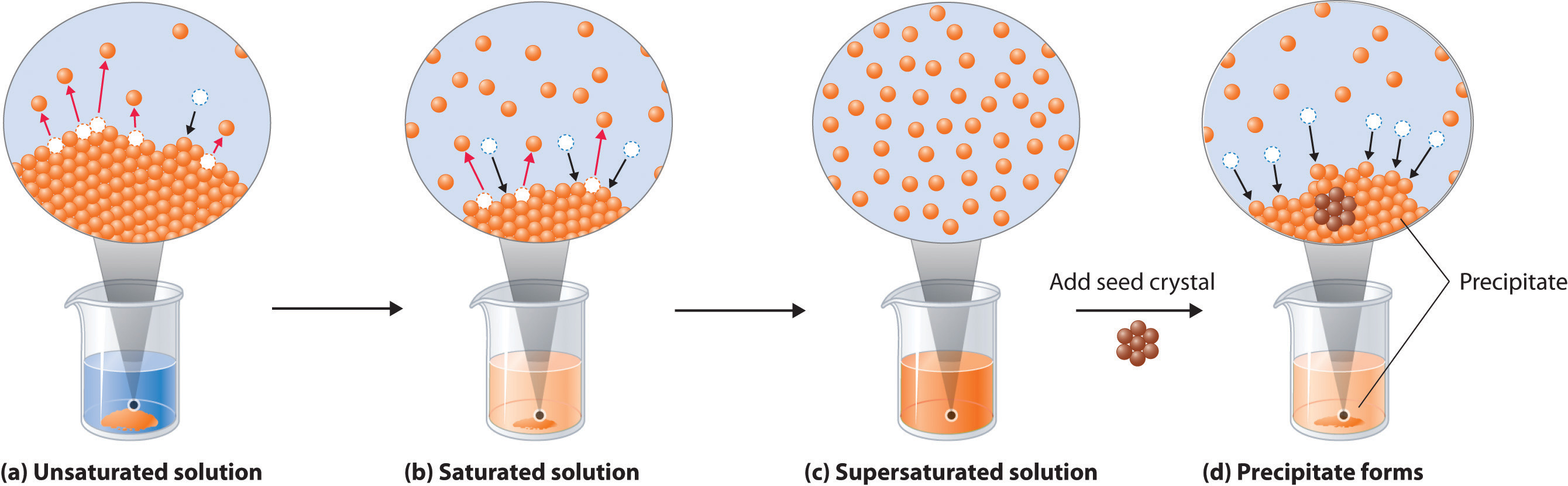
Effigy 9.2.ane Dissolution and Precipitation (a) When a solid is added to a solvent in which information technology is soluble, solute particles go out the surface of the solid and become solvated by the solvent, initially forming an unsaturated solution. (b) When the maximum possible amount of solute has dissolved, the solution becomes saturated. If excess solute is present, the rate at which solute particles get out the surface of the solid equals the rate at which they return to the surface of the solid. (c) A supersaturated solution can usually exist formed from a saturated solution past filtering off the excess solute and lowering the temperature. (d) When a seed crystal of the solute is added to a supersaturated solution, solute particles leave the solution and form a crystalline precipitate.
Factors Affecting Solubility
The maximum amount of a solute that can dissolve in a solvent at a specified temperature and force per unit area is its solubilityA measure of the how much of a solid substance remains dissolved in a given amount of a specified liquid at a specified temperature and pressure level. . Solubility is oftentimes expressed as the mass of solute per book (g/50) or mass of solute per mass of solvent (g/chiliad), or every bit the moles of solute per book (mol/50). Fifty-fifty for very soluble substances, however, there is usually a limit to how much solute can dissolve in a given quantity of solvent. In general, the solubility of a substance depends on not just the energetic factors we have discussed but also the temperature and, for gases, the pressure. At 20°C, for example, 177 thousand of NaI, 91.2 chiliad of NaBr, 35.ix g of NaCl, and just 4.1 g of NaF dissolve in 100 g of h2o. At 70°C, yet, the solubilities increment to 295 g of NaI, 119 g of NaBr, 37.v k of NaCl, and iv.8 yard of NaF. Equally you learned in Affiliate 4, the lattice energies of the sodium halides increment from NaI to NaF..
A solution with the maximum possible amount of solute is saturatedA solution with the maximum possible amount of a solute nether a given set of conditions. . If a solution contains less than the maximum amount of solute, it is unsaturated. When a solution is saturated and excess solute is nowadays, the charge per unit of dissolution is exactly equal to the rate of crystallization (part (b) in Effigy 9.1.1). Using the value just stated, a saturated aqueous solution of NaCl, for instance, contains 35.9 one thousand of NaCl per 100 mL of water at 20°C. We can fix a homogeneous saturated solution past adding excess solute (in this case, greater than 35.9 yard of NaCl) to the solvent (water), stirring until the maximum possible amount of solute has dissolved, and then removing undissolved solute by filtration.
Note the Blueprint
The solubility of most solids increases with increasing temperature.
Because the solubility of near solids increases with increasing temperature, a saturated solution that was prepared at a higher temperature usually contains more dissolved solute than it would comprise at a lower temperature. When the solution is cooled, it can therefore become supersaturatedAn unstable solution with more than dissolved solute than it would ordinarily contain under the given prepare of conditions. (part (c) in Figure 9.1.one). Similar a supercooled or superheated liquid, a supersaturated solution is unstable. Consequently, adding a small particle of the solute, a seed crystalA solid sample of a substance that can be added to a supercooled liquid or a supersaturated solution to help induce crystallization. , will usually cause the excess solute to speedily precipitate or crystallize, sometimes with spectacular results.
Effigy nine.2.2 Crystallization of Sodium Acetate out of a Supersaturated Solution: The crystallization of sodium acetate out of a supersaturated solution can exist spectacular. In this video from Mike Shin a small seed crystal is introduced into the middle of the solution
If the rate of crystallization in Equation ix.2.1 is greater than the rate of dissolution, crystals or a precipitate will form (role (d) in Effigy 9.2.1). In contrast, calculation a seed crystal to a saturated solution reestablishes the dynamic equilibrium, and the net quantity of dissolved solute no longer changes.
Interactions in Liquid Solutions
The interactions that make up one's mind the solubility of a substance in a liquid depend largely on the chemic nature of the solute (such as whether information technology is ionic or molecular) rather than on its physical land (solid, liquid, or gas). We will showtime draw the general example of forming a solution of a molecular species in a liquid solvent and then draw the formation of a solution of an ionic compound. We postpone until Chapters 16 and 17 deeper analysis of the equilibria betwixt phases based on thermodynamics.
Solutions of Molecular Substances in Liquids
The London dispersion forces, dipole–dipole interactions, and hydrogen bonds that hold molecules to other molecules are more often than not weak. Still, free energy is required to disrupt these interactions.
For solutions of gases in liquids, we can safely ignore the free energy required to separate the solute molecules considering the molecules in the gas stage are already separated. Thus nosotros need to consider merely the free energy required to split up the solvent molecules and the free energy released by new solute–solvent interactions.
Nonpolar gases such as N2, Oii, and Ar take no dipole moment and cannot appoint in dipole–dipole interactions or hydrogen bonding. Consequently, the only way they can interact with a solvent is past means of London dispersion forces, which may be weaker than the solvent–solvent interactions in a polar solvent. It is not surprising, then, that nonpolar gases are most soluble in nonpolar solvents. The interactions between the solvent molecules and the solvent-solute interactions are both London dispersion forces and of roughly equal size.
When solvent-solvent and solvent-solute interactions are the same one calls the solution ideal. In an ideal gas, the molecules do not interact at all. In an platonic liquid the molecules must interact to concord the fluid together, but the interaction between the solvent molecules and those betwixt the solvent and solute molecules are the same.
In contrast, for a solution of a nonpolar gas in a polar solvent, the interaction of the polar solvent molecules is far greater than the interaction of the polar solvent molecules with the not-polar solute molecules. As a result, nonpolar gases are less soluble in polar solvents than in nonpolar solvents. For example, the concentration of N2 in a saturated solution of N2 in water, a polar solvent, is only seven.07 × 10−4 M compared with 4.v × ten−3 M for a saturated solution of Northii in benzene, a nonpolar solvent.
The solubilities of nonpolar gases in water generally increase as the molecular mass of the gas increases, equally shown in Table ix.2.1 This is precisely the tendency expected: as the gas molecules go larger, the forcefulness of the solvent–solute interactions due to London dispersion forces increases, budgeted the force of the solvent–solvent interactions.
Tabular array nine.2.1 Solubilities of Selected Gases in Water at twenty°C and 1 atm Pressure
| Gas | Solubility (M) × 10−four |
|---|---|
| He | 3.90 |
| Ne | 4.65 |
| Ar | fifteen.2 |
| Kr | 27.ix |
| Xe | 50.ii |
| H2 | 8.06 |
| N2 | vii.07 |
| CO | 10.6 |
| O2 | 13.nine |
| North2O | 281 |
| CHfour | fifteen.5 |
Nearly all mutual organic liquids, whether polar or not, are miscible. The strengths of the intermolecular attractions are comparable; and the solutions are shut to ideal. Another factor, which we will discuss in Chapter 17, the increase in disorder (entropy), drives the germination of a solution. If the predominant intermolecular interactions in 2 liquids are very dissimilar from 1 another, yet, they may be immiscible. For example, organic liquids such as benzene, hexane, CCl4, and CStwo (Southward=C=S) are nonpolar and accept no ability to act every bit hydrogen bond donors or acceptors with hydrogen-bonding solvents such equally H2O, HF, and NH3; hence they are immiscible in these solvents. When shaken with water, they form separate phases or layers separated by an interface (Effigy nine.ii.three), the region betwixt the two layers. Just because two liquids are immiscible, however, does not hateful that they are completely insoluble in each other. For instance, 188 mg of benzene dissolves in 100 mL of h2o at 23.5°C. Adding more benzene results in the separation of an upper layer consisting of benzene with a small amount of dissolved water (the solubility of water in benzene is only 178 mg/100 mL of benzene).

Effigy ix.ii.3 Water is immiscible with with perfluoroheptane (and about halogenated compounds). Because water is less dense than the perfluoroheptane, the water layer floats on top. The goldfish is swimming in the water layer. Figure from the Wikipedia..
The solubilities of simple alcohols in water are given in Tabular array 9.two.2. But the 3 lightest alcohols (methanol, ethanol, and n-propanol) are completely miscible with h2o. As the molecular mass of the alcohol increases, then does the proportion of hydrocarbon in the molecule. Correspondingly, the importance of hydrogen bonding and dipole–dipole interactions in the pure booze decreases, while the importance of London dispersion forces increases, which leads to progressively fewer favorable electrostatic interactions with water. Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with h2o yet sufficiently nonpolar to exist completely miscible with all organic solvents.
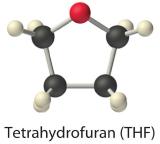
Table 9.ii.two Solubilities of Direct-Chain Organic Alcohols in H2o at 20°C
| Booze | Solubility (mol/100 grand of H2O) |
|---|---|
| methanol | completely miscible |
| ethanol | completely miscible |
| n-propanol | completely miscible |
| n-butanol | 0.eleven |
| due north-pentanol | 0.030 |
| n-hexanol | 0.0058 |
| n-heptanol | 0.0008 |
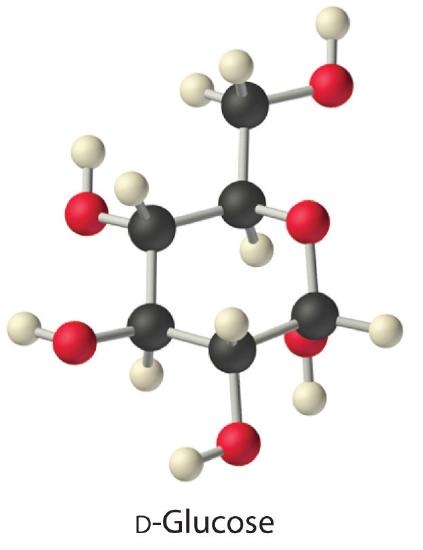
The aforementioned principles govern the solubilities of molecular solids in liquids. For example, elemental sulfur is a solid consisting of cyclic S8 molecules that accept no dipole moment. Because the Southwardeight rings in solid sulfur are held to other rings by London dispersion forces, elemental sulfur is insoluble in water. It is, however, soluble in nonpolar solvents that have comparable London dispersion forces, such as CS2 (23 one thousand/100 mL). In contrast, glucose contains five –OH groups that can form hydrogen bonds. Consequently, glucose is very soluble in water (91 g/120 mL of water) but essentially insoluble in nonpolar solvents such every bit benzene. The structure of one isomer of glucose is shown hither.
Low-molecular-mass hydrocarbons with highly electronegative and polarizable halogen atoms, such as chloroform (CHClthree) and methylene chloride (CHiiCl2), have both significant dipole moments and relatively strong London dispersion forces. These hydrocarbons are therefore powerful solvents for a wide range of polar and nonpolar compounds. Naphthalene, which is nonpolar, and phenol (C6H5OH), which is polar, are very soluble in chloroform. In contrast, the solubility of ionic compounds is largely adamant not by the polarity of the solvent but rather by its dielectric constant, a measure of its ability to dissever ions in solution, as you will soon encounter.
Example 9.ii.ane
Identify the most important solute–solvent interactions in each solution.
- iodine in benzene
-
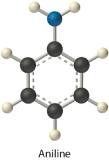
aniline (CviH5NH2) in dichloromethane (CH2Clii)
- iodine in water
Given: components of solutions
Asked for: predominant solute–solvent interactions
Strategy:
Identify all possible intermolecular interactions for both the solute and the solvent: London dispersion forces, dipole–dipole interactions, or hydrogen bonding. Determine which is likely to be the most of import factor in solution germination.
Solution:
- Benzene and Iii are both nonpolar molecules. The just possible attractive forces are London dispersion forces.
- Aniline is a polar molecule with an –NH2 group, which can human action equally a hydrogen bond donor. Dichloromethane is likewise polar, merely it has no obvious hydrogen bond acceptor. Therefore, the nearly important interactions between aniline and CHtwoCltwo are likely to be London interactions.
- Water is a highly polar molecule that engages in extensive hydrogen bonding, whereas Itwo is a nonpolar molecule that cannot act as a hydrogen bond donor or acceptor. The slight solubility of Iii in water (1.3 × ten−3 mol/L at 25°C) is due to London dispersion forces.
Exercise
Place the most of import interactions in each solution:
- ethylene glycol (HOCH2CH2OH) in acetone
- acetonitrile (CHiiiC≡N) in acetone
- n-hexane in benzene
Answer:
- hydrogen bonding
- London interactions
- London dispersion forces
Hydrophilic and Hydrophobic Solutes
A solute tin can be classified every bit hydrophilicA substance attracted to water. Hydrophilic substances are polar and can form hydrogen bond 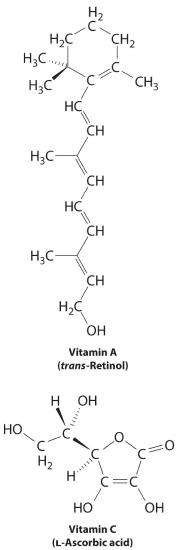 south to h2o. (literally, "h2o loving"), significant that it has an electrostatic allure to water, or hydrophobicA substance that repels water. Hydrophobic substances practise not interact favorably with h2o. ("water fearing"), meaning that it repels h2o. A hydrophilic substance is polar and often contains O–H or North–H groups that can grade hydrogen bonds to water. For case, glucose with its five O–H groups is hydrophilic. In contrast, a hydrophobic substance may exist polar merely usually contains C–H bonds that exercise non collaborate favorably with water, as is the case with naphthalene and northward-octane. Hydrophilic substances tend to exist very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in h2o and soluble in nonpolar solvents such as benzene and cyclohexane.
south to h2o. (literally, "h2o loving"), significant that it has an electrostatic allure to water, or hydrophobicA substance that repels water. Hydrophobic substances practise not interact favorably with h2o. ("water fearing"), meaning that it repels h2o. A hydrophilic substance is polar and often contains O–H or North–H groups that can grade hydrogen bonds to water. For case, glucose with its five O–H groups is hydrophilic. In contrast, a hydrophobic substance may exist polar merely usually contains C–H bonds that exercise non collaborate favorably with water, as is the case with naphthalene and northward-octane. Hydrophilic substances tend to exist very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in h2o and soluble in nonpolar solvents such as benzene and cyclohexane.
The difference between hydrophilic and hydrophobic substances has substantial consequences in biological systems. For example, vitamins can be classified as either fatty soluble or water soluble. Fat-soluble vitamins, such as vitamin A, are mostly nonpolar, hydrophobic molecules. As a consequence, they tend to be absorbed into fatty tissues and stored there. In dissimilarity, h2o-soluble vitamins, such as vitamin C, are polar, hydrophilic molecules that circulate in the blood and intracellular fluids, which are primarily aqueous. H2o-soluble vitamins are therefore excreted much more than speedily from the trunk and must be replenished in our daily diet. A comparison of the chemic structures of vitamin A and vitamin C rapidly reveals why i is hydrophobic and the other hydrophilic.
Because water-soluble vitamins are apace excreted, the chance of consuming them in excess is relatively small. Eating a dozen oranges a twenty-four hours is probable to make you tired of oranges long before y'all suffer any ill furnishings due to their high vitamin C content. In contrast, fatty-soluble vitamins constitute a meaning health gamble when consumed in large amounts. For instance, the livers of polar bears and other large animals that live in cold climates comprise big amounts of vitamin A, which take occasionally proven fatal to humans who have eaten them.
Example nine.two.2
The post-obit substances are essential components of the human nutrition:
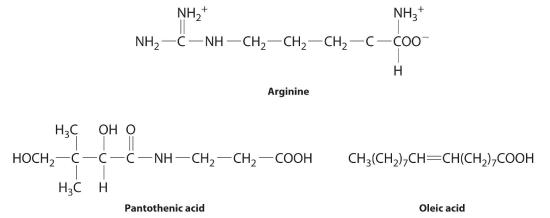
Using what you lot know of hydrophilic and hydrophobic solutes, allocate each as water soluble or fatty soluble and predict which are likely to be required in the diet on a daily ground.
- arginine
- pantothenic acid
- oleic acid
Given: chemical structures
Asked for: nomenclature every bit h2o soluble or fat soluble; dietary requirement
Strategy:
Based on the structure of each compound, make up one's mind whether it is hydrophilic or hydrophobic. If it is hydrophilic, it is probable to be required on a daily basis.
Solution:
- Arginine is a highly polar molecule with two positively charged groups and one negatively charged group, all of which tin can form hydrogen bonds with water. As a result, information technology is hydrophilic and required in our daily diet.
- Although pantothenic acid contains a hydrophobic hydrocarbon portion, it also contains several polar functional groups (–OH and –CO2H) that should collaborate strongly with h2o. It is therefore probable to exist h2o soluble and required in the nutrition. (In fact, pantothenic acid is almost always a component of multiple-vitamin tablets.)
- Oleic acid is a hydrophobic molecule with a single polar grouping at one end. It should be fat soluble and not required daily.
Practise
These compounds are consumed by humans: caffeine, acetaminophen, and vitamin D. Identify each every bit primarily hydrophilic (water soluble) or hydrophobic (fat soluble), and predict whether each is likely to be excreted from the body rapidly or slowly.
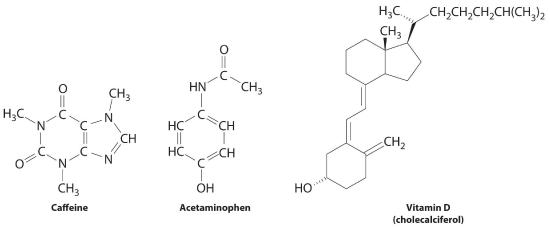
Respond: Caffeine and acetaminophen are water soluble and rapidly excreted, whereas vitamin D is fat soluble and slowly excreted.
Solid Solutions
Solutions are not limited to gases and liquids; solid solutions too exist. For instance, amalgams are solutions of metals in liquid mercury. Because nearly metals are soluble in mercury, amalgams are used in golden mining, dentistry, and many other applications. A major difficulty when mining gilt is separating very small particles of pure gold from tons of crushed stone. One way to achieve this is to agitate a suspension of the crushed rock with liquid mercury, which dissolves the gold (also equally any metallic silver that might be nowadays). The very dense liquid gold–mercury amalgam is then isolated and the mercury distilled abroad.
An blend is a solid or liquid solution that consists of one or more elements in a metallic matrix. A solid blend has a single homogeneous phase in which the crystal construction of the solvent remains unchanged by the presence of the solute. Thus the microstructure of the alloy is uniform throughout the sample. Examples are substitutional and interstitial alloys such as contumely or solder. (For more information virtually alloys, come across Section 8.5) Liquid alloys include sodium/potassium and gold/mercury. In contrast, a fractional blend solution has ii or more phases that can be homogeneous in the distribution of the components, but the microstructures of the two phases are not the same. As a liquid solution of lead and can is cooled, for example, dissimilar crystalline phases form at dissimilar cooling temperatures. As yous learned in Section 8.5, alloys ordinarily have properties that differ from those of the component elements.
Network solids such as diamond, graphite, and SiO2 are insoluble in all solvents with which they do not react chemically. The covalent bonds that hold the network or lattice together are merely too strong to be broken under normal conditions. They are certainly much stronger than any conceivable combination of intermolecular interactions that might occur in solution. About metals are insoluble in nearly all solvents for the same reason: the delocalized metal bonding is much stronger than any favorable metal atom–solvent interactions. Many metals react with solutions such as aqueous acids or bases to produce a solution. However, as we saw in these instances the metal undergoes a chemical transformation that cannot be reversed past simply removing the solvent.
Notation the Pattern
Solids with very strong intermolecular bonding tend to exist insoluble.
Solubilities of Ionic Substances in Liquids
Ionic substances are by and large almost soluble in polar solvents; the higher the lattice energy, the more polar the solvent must be to overcome the lattice free energy and dissolve the substance. Because of its high polarity, water is the most mutual solvent for ionic compounds. Many ionic compounds are soluble in other polar solvents, nonetheless, such as liquid ammonia, liquid hydrogen fluoride, and methanol. Because all these solvents consist of molecules that take relatively large dipole moments, they can interact favorably with the dissolved ions.
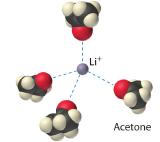
The interaction of water with Na+ and Cl− ions in an aqueous solution of NaCl is shown whenever you sprinkle salt into h2o, for example when y'all are cooking. The ion–dipole interactions betwixt Li+ ions and acetone molecules in a solution of LiCl in acetone are shown in Figure ix.ii.iv The energetically favorable Li+–acetone interactions make the solvent solute interaction sufficiently negative to overcome the positive lattice energy of the LiCl and the acetone-acetone interactions. Considering the dipole moment of acetone (two.88 D), and thus its polarity, is actually larger than that of water (1.85 D), 1 might even expect that LiCl would be more than soluble in acetone than in water. In fact, the contrary is true: 83 g of LiCl dissolve in 100 mL of h2o at 20°C, merely merely well-nigh iv.one 1000 of LiCl dissolve in 100 mL of acetone. This apparent contradiction arises from the fact that the dipole moment is a property of a single molecule in the gas phase. A more than useful mensurate of the power of a solvent to dissolve ionic compounds is its dielectric abiding (ε) which is the ability of a majority substance to decrease the electrostatic forces between two charged particles. By definition, the dielectric constant of a vacuum is 1. In essence, a solvent with a high dielectric abiding causes the charged particles to carry equally if they have been moved farther autonomously. At 25°C, the dielectric abiding of water is eighty.1, one of the highest known, and that of acetone is only 21.0. Hence water is meliorate able to decrease the electrostatic attraction between Li+ and Cl− ions, so LiCl is more than soluble in water than in acetone. This behavior is in dissimilarity to that of molecular substances, for which polarity is the dominant factor governing solubility.
Note the Pattern
A solvent's dielectric constant is the nigh useful measure of its ability to dissolve ionic compounds. A solvent's polarity is the dominant factor in dissolving molecular substances.
Figure nine.2.four Ion–Dipole Interactions in the Solvation of Li+ Ions past Acetone, a Polar Solvent
Information technology is likewise possible to dissolve ionic compounds in organic solvents using crown ethersCyclic polyether with 4 or more oxygen atoms separated past two or 3 carbon atoms. All crown ethers have a central cavity that tin can accommodate a metal ion coordinated to the ring of oxygen atoms. , cyclic compounds with the general formula (OCH2CH2) n . Crown ethers are named using both the total number of atoms in the ring and the number of oxygen atoms. Thus 18-crown-six is an xviii-membered ring with six oxygen atoms (function (a) in Figure ix.2.5 ). The cavity in the center of the crown ether molecule is lined with oxygen atoms and is large enough to exist occupied by a cation, such every bit K+. The cation is stabilized by interacting with lone pairs of electrons on the surrounding oxygen atoms. Thus crown ethers solvate cations inside a hydrophilic cavity, whereas the outer beat out, consisting of C–H bonds, is hydrophobic. Crown ethers are useful for dissolving ionic substances such as KMnO4 in organic solvents such as isopropanol [(CH3)2CHOH] (Figure nine.ii.5). The availability of crown ethers with cavities of different sizes allows specific cations to exist solvated with a high caste of selectivity.
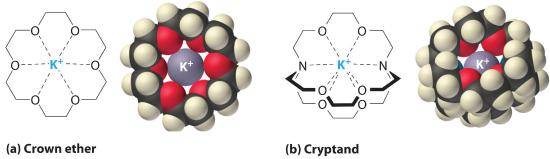
Figure 9.2.5 Crown Ethers and Cryptands (a) The potassium complex of the crown ether xviii-crown-vi. Annotation how the cation is nestled inside the cardinal cavity of the molecule and interacts with lone pairs of electrons on the oxygen atoms. (b) The potassium circuitous of 2,2,2-cryptand, showing how the cation is almost subconscious past the cryptand. Cryptands solvate cations via lone pairs of electrons on both oxygen and nitrogen atoms.
Figure 9.2.6. Effect of a Crown Ether on the Solubility of KMnOiv in Benzene. Commonly which is intensely imperial, is completely insoluble in benzene which has a relatively low dielectric constant. In the presence of a minor corporeality of crown ether, KMnO4 dissolves in benzene every bit shown by the reddish imperial color acquired by the permanganate ions in solution. Video from Chem Toddler
Cryptands Consisting of three (OCH) 2 CH 2 O–)n bondage connected by two nitrogen atoms, cryptands have a fundamental cavity that can encapsulate a metal ion coordinated to the oxygen and nitrogen atoms. (from the Greek kryptós, significant "hidden") are compounds that can completely surround a cation with solitary pairs of electrons on oxygen and nitrogen atoms (office (b) in Figure 9.2.5). The number in the name of the cryptand is the number of oxygen atoms in each strand of the molecule. Similar crown ethers, cryptands can exist used to set solutions of ionic compounds in solvents that are otherwise too nonpolar to dissolve them.
Summary
The solubility of a substance is the maximum amount of a solute that tin deliquesce in a given quantity of solvent; it depends on the chemical nature of both the solute and the solvent and on the temperature and pressure level. When a solution contains the maximum amount of solute that can dissolve nether a given set of conditions, it is a saturated solution. Otherwise, it is unsaturated. Supersaturated solutions, which contain more dissolved solute than immune under detail atmospheric condition, are not stable; the addition of a seed crystal, a small particle of solute, volition unremarkably cause the excess solute to crystallize. A system in which crystallization and dissolution occur at the same rate is in dynamic equilibrium. The solubility of a substance in a liquid is determined by intermolecular interactions, which also determine whether two liquids are miscible. Solutes can be classified equally hydrophilic (water loving) or hydrophobic (water fearing). Vitamins with hydrophilic structures are water soluble, whereas those with hydrophobic structures are fatty soluble. Many metals dissolve in liquid mercury to form amalgams. Covalent network solids and well-nigh metals are insoluble in most all solvents. The solubility of ionic compounds is largely adamant by the dielectric constant (ε) of the solvent, a measure out of its ability to decrease the electrostatic forces between charged particles. Solutions of many ionic compounds in organic solvents tin be dissolved using crown ethers, cyclic polyethers large enough to arrange a metallic ion in the middle, or cryptands, compounds that completely environment a cation.
Cardinal Takeaway
- The force of intramolecular bonding determines the solubility of a solute in a given solvent.
Conceptual Problems
-
If a compound is only slightly soluble in a particular solvent, what are the relative strengths of the solvent–solvent and solute–solute interactions versus the solute–solvent interactions?
-
Predict whether each of the following sets of conditions favors formation of a solution:
Intermolecular Attractive Forces (Solute) Intermolecular Attractive Forces (Solvent) ΔH soln London dispersion hydrogen bonding slightly positive dipole–dipole hydrogen bonding very negative ionic dipole–dipole slightly positive ionic London dispersion positive -
Suit the following liquids in order of increasing solubility in h2o: t-butanol [(CHthree)iiiCOH], benzene, ammonia, and heptane. Justify your answer.
-
Which compound in each pair volition be more soluble in h2o? Explain your reasoning in each example.
- toluene (C7Hviii) or ethyl ether (CtwoHvOCiiHfive)
- chloroform (CHCl3) or acetone (CH3COCH3)
- carbon tetrachloride (CCl4) or tetrahydrofuran (C4H8O)
- CaCl2 or CH2Cl2
-
Which chemical compound in each pair will exist more soluble in benzene? Explicate your reasoning in each instance.
- cyclohexane or methanol
- I2 or MgCl2
- methylene chloride (CH2Clii) or acerb acid
-
Two water-insoluble compounds—north-decylamine [CH3(CH2)9NHtwo] and n-decane—tin be separated by the post-obit procedure: The compounds are dissolved in a solvent such as toluene that is immiscible with water. When adding an aqueous HCl solution to the mixture and stirring vigorously, the HCl reacts with ane of the compounds to produce a common salt. When the stirring is stopped and the mixture is allowed to stand, ii layers are formed. At this bespeak, each layer contains merely i of the 2 original compounds. After the layers are separated, adding aqueous NaOH to the aqueous layer liberates one of the original compounds, which can and then be removed past stirring with a second portion of toluene to excerpt it from the h2o.
- Place the compound that is present in each layer following the addition of HCl. Explain your reasoning.
- How tin can the original compounds be recovered from the toluene solution?
-
Bromine and iodine are both soluble in CCliv, but bromine is much more than soluble. Why?
-
A solution is made by mixing 50.0 mL of liquid A with 75.0 mL of liquid B. Which is the solute, and which is the solvent? Is information technology valid to assume that the volume of the resulting solution will be 125 mL? Explain your answer.
-
The compounds NaI, NaBr, and NaCl are far more soluble in water than NaF, a substance that is used to fluoridate drinking water. In fact, at 25°C the solubility of NaI is 184 1000/100 mL of water, versus only 4.2 m/100 mL of water for NaF. Why is sodium iodide so much more soluble in h2o? Do you look KCl to be more soluble or less soluble in water than NaCl?
-
When h2o is mixed with a solvent with which information technology is immiscible, the two liquids usually grade ii divide layers. If the density of the nonaqueous solvent is 1.75 chiliad/mL at room temperature, sketch the advent of the heterogeneous mixture in a beaker and label which layer is which. If you were not certain of the density and the identity of the other liquid, how might you lot be able to place which is the aqueous layer?
-
When two liquids are immiscible, the improver of a third liquid tin occasionally be used to induce the formation of a homogeneous solution containing all iii.
- Ethylene glycol (HOCHtwoCH2OH) and hexane are immiscible, just calculation acetone [(CH3)2CO] produces a homogeneous solution. Why does adding a third solvent produce a homogeneous solution?
- Methanol and n-hexane are immiscible. Which of the following solvents would y'all add to create a homogeneous solution—water, due north-butanol, or cyclohexane? Justify your choice.
-
Some proponents of vitamin therapy for combating illness encourage the consumption of large amounts of fatty-soluble vitamins. Why can this be dangerous? Would it be as dangerous to swallow large amounts of water-soluble vitamins? Why or why not?
-
Why are about metals insoluble in virtually all solvents?
-
Because sodium reacts violently with h2o, it is difficult to weigh out small quantities of sodium metal for a reaction due to its rapid reaction with minor amounts of moisture in the air. Would a Na/Hg amalgam be every bit sensitive to moisture as metallic sodium? Why or why not? A Na/K blend is a liquid at room temperature. Will it exist more or less sensitive to moisture than solid Na or Thousand?
-
Dental amalgams often contain high concentrations of Hg, which is highly toxic. Why isn't dental amalgam toxic?
-
Arrange two,ii,3-trimethylpentane, one-propanol, toluene (C7H8), and dimethyl sulfoxide [(CH3)2S=O] in gild of increasing dipole moment. Explain your reasoning.
-
Conform acetone, chloroform, cyclohexane, and ii-butanol in order of increasing dielectric constant. Explain your reasoning.
-
Dissolving a white crystalline chemical compound in ethanol gave a blue solution. Evaporating the ethanol from the solution gave a bluish-crystalline product, which slowly transformed into the original white solid on standing in the air for several days. Explain what happened. How does the mass of the initial bluish solid compare with the mass of the white solid finally recovered?
-
You lot have been asked to develop a new drug that could be used to bind Feiii+ ions in patients who suffer from atomic number 26 toxicity, allowing the bound iron to exist excreted in the urine. Would you consider a crown ether or a cryptand to be a reasonable candidate for such a drug? Explain your respond.
-
Describe two different situations in which fractional crystallization will non work as a separation technique when attempting to isolate a single compound from a mixture.
-
You have been given a mixture of 2 compounds—A and B—and accept been told to isolate pure A. Y'all know that pure A has a lower solubility than pure B and that the solubilities of both A and B increment with temperature. Outline a process to isolate pure A. If B had the lower solubility, could y'all use the same procedure to isolate pure A? Why or why not?
Answers
-
London dispersion forces increase with increasing diminutive mass. Iodine is a solid while bromine is a liquid due to the greater intermolecular interactions between the heavier iodine atoms. Iodine is less soluble than bromine in virtually all solvents because it requires more than energy to dissever I2 molecules than Brtwo molecules.
-
- A tertiary solvent with intermediate polarity and/or dielectric constant can finer dissolve both of the immiscible solvents, creating a single liquid phase.
- n-butanol—it is intermediate in polarity between methanol and northward-hexane, while h2o is more than polar than either and cyclohexane is comparable to n-hexane.
-
In dental amalgam, the mercury atoms are locked in a solid stage that does not undergo corrosion under physiological weather condition; hence, the mercury atoms cannot readily diffuse to the surface where they could vaporize or undergo chemical reaction.
-
Deliquesce the mixture of A and B in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off whatsoever undissolved B, and absurd slowly. Pure A should crystallize, while B stays in solution. If B were less soluble, information technology would be impossible to obtain pure A by this method in a single footstep, because some of the less soluble compound (B) will ever exist present in the solid that crystallizes from solution.
Are Hexane And Benzene Miscible,
Source: https://chem.libretexts.org/Courses/Prince_Georges_Community_College/CHEM_2000:_Chemistry_for_Engineers_%28Sinex%29/Unit_3:_States_of_Matter/Chapter_9:_Solutions/Chapter_9.2:_Solubility_and_Structure
Posted by: hamelwithris.blogspot.com


0 Response to "Are Hexane And Benzene Miscible"
Post a Comment